T Cell Testing Service
ImmunoServ offers a truly scalable T cell testing service for measuring COVID-19 immunity in large cohorts and population studies.
Our scientists are experts in the T cell immunology field, with more than 100 publications measuring T cell responses to viruses, bacteria and cancer.
Drawing on our expertise in measuring adaptive immune responses to viruses, ImmunoServ created the COVID-19 Immuno-T™ test, a simple and scalable whole blood-based assay for measuring T cell immune responses to SARS-CoV-2. To learn more about how the COVID-19 Immuno-T test works click here.
We provide an end-to-end service including blood sample collection, UN3373 compliant logistics, full analysis, and data interpretation.
Our Clients
ImmunoServ's T cell testing service is supporting several public health bodies and research institutes providing significant insight into COVID-19 immunity.
We are also developing a range of T cell tests for other diseases, including influenza, cancer and autoimmunity.
If you are interested in using our T cell testing service, please contact us.

Our Impact during the COVID-19 pandemic
Throughout the COVID-19 pandemic, ImmunoServ’s COVID-19 Immuno-T™ test has been performed on >1000 patient and participant blood samples across multiple research studies.
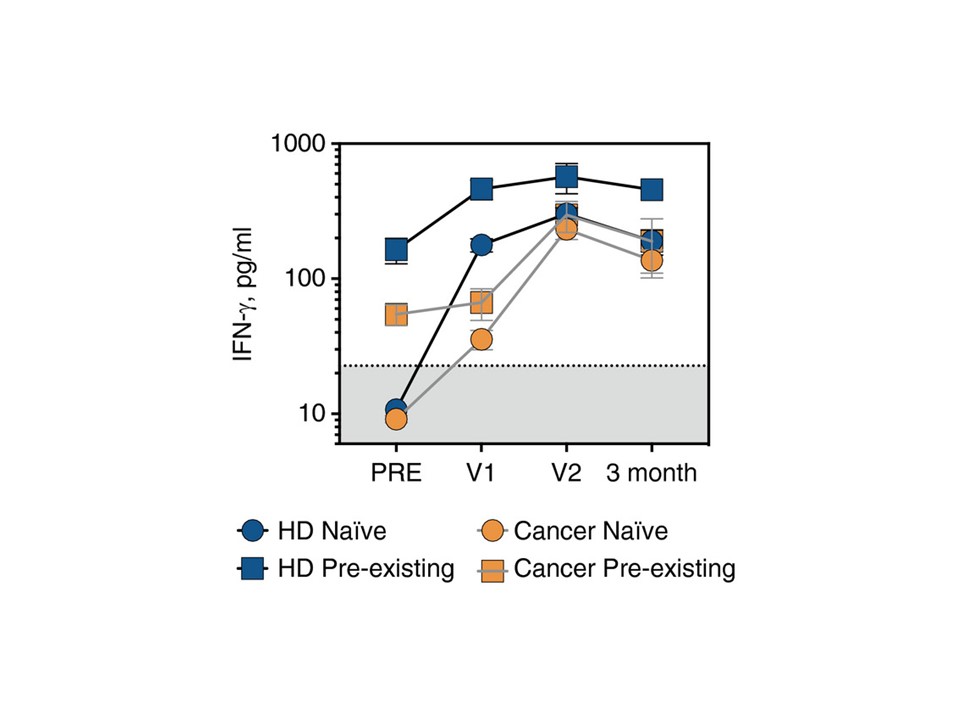
The COVID Immune study, funded by Health and Care Research Wales and the UK government, examined the effect of COVID-19 vaccination on both SARS-CoV-2-specific T cell and antibody responses in cancer patients. This joint project, run by Cardiff University, Velindre NHS Cancer Centre and the Wales Cancer Bank, utilised the COVID-19 Immuno-T™ test to measure SARS-CoV-2 T cell responses induced by COVID-19 vaccination in 68 cancer patients. Results published in Immunology revealed that the vast majority of cancer patients do mount robust immunological responses following at least two doses of COVID-19 vaccination, regardless of tumour type, stage or treatment.
The COVID-19 National Core Studies Immunity (NCSi) programme, funded by UK Research and Innovation, identified a number of areas where additional resource was needed to respond to urgent unanswered questions about COVID-19. The COVID-19 Immuno-T™ test was incorporated into multiple studies, including EVITE Immunity (‘Investigating the effects of shielding in Wales’, led by Prof Helen Snooks, Swansea University), Asymptomatic COVID-19 in Education (ACE) (‘Studying asymptomatic cases of COVID-19 in student populations to understand protective immunity’, led by Prof Lucy Fairclough, University of Nottingham) and Vaccine Response in People with Chronic Lymphocytic Leukaemia (‘Establishing effectiveness of the COVID-19 vaccines in people with CLL’, led by Dr Helen Parry, University of Birmingham). Download the full NCSi Impact Report here. Together, these studies enabled the UK to use health data and research to inform government responses to COVID-19.

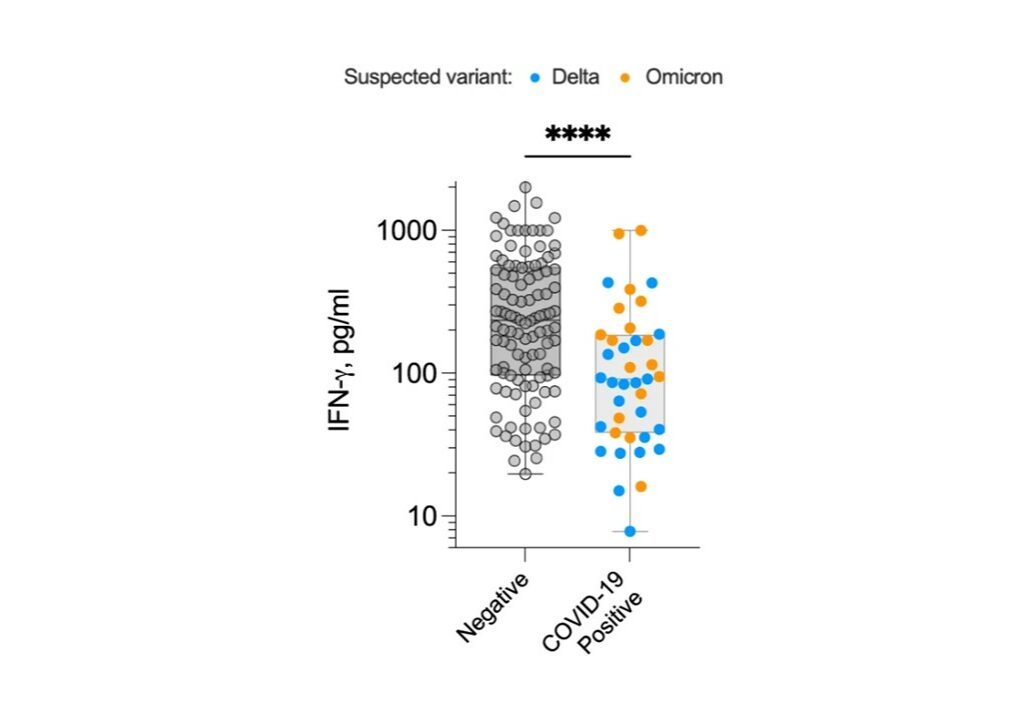
The SARS-CoV-2 T cell response test study, funded by Innovate UK and led by Dr Martin Scurr (Cardiff University, ImmunoServ Ltd.), developed a novel capillary blood T cell assay for large scale population immunity assessments. Over 300 participants were recruited across the UK within 10 weeks at the height of the initial SARS-CoV-2 Omicron wave, with the results published in Nature Communications demonstrating that the magnitude of T cell responsiveness to SARS-CoV-2 is an effective correlate of protection against developing COVID-19. The study led to the successful commercialisation of the finger prick version of ImmunoServ’s COVID-19 Immuno-T™ test, primarily being used by immune compromised individuals concerned about their immunity status. Now anyone in the UK can measure their T cell immunity to COVID-19 through our consumer site Immuno-T.co.uk.
COVID-19 Vaccine Response in People with MS studies, funded by the BMA Foundation for Medical Research and led by Prof Ruth Dobson (Queen Mary University of London) and Dr Emma Tallantyre (Cardiff University, Cardiff & Vale NHS) utilised COVID-19 Immuno-T™ on blood samples obtained from MS patients recruited across five UK MS centers (Cardiff, Newport, Nottingham, Royal London Hospital [Barts Health NHS Trust] and Swansea). Two highly cited seminal studies (1, 2) have been reported that demonstrate the effect of MS treatments on COVID-19 vaccine-induced T cell and antibody responses, revealing the potential protective effect of SARS-CoV-2-specific T cell responses in seronegative subjects.

Now Available
At ImmunoServ we are on a mission to bring T cell testing to the masses. The World's first At-Home COVID-19 T cell test is available to anyone in the United Kingdom.
If you would like to test your own T cell immune response to COVID-19 using the At Home Immuno-T test, please visit our Immuno-T website.
The At Home Immuno-T test is available across the UK for only £79.
References
1. Scurr MJ et al. (2022). Magnitude of venous or capillary blood-derived SARS-CoV-2-specific T cell response determines COVID-19 immunity. Nature Communications.
2. Scurr MJ et al. (2021). Whole blood‐based measurement of SARS‐CoV‐2‐specific T cells reveals asymptomatic infection and vaccine immunogenicity in healthy subjects and patients with solid‐organ cancers. Immunology.
3. Tallantyre E, Vickaryous N, et al. (2021). COVID‐19 Vaccine Response in People with Multiple Sclerosis. Annals of Neurology.
4. Ponsford M, et al. (2021). Persistent COVID-19 Infection in Wiskott-Aldrich Syndrome Cleared Following Therapeutic Vaccination: a Case Report. Journal of Clinical Immunology.